- Resources
Recent Activities
Plants Gallery
Downloads
Worldwide, there are plants known as psychoactive plants that naturally contain psychedelic active components. They have a high concentration of neuroprotective substances that can interact with the nervous system to produce psychedelic effects. Despite these plants' hazardous potential, recreational use of them is on the rise because of their psychoactive properties. Early neuroscience studies relied heavily on psychoactive plants and plant natural products (NPs), and both recreational and hazardous NPs have contributed significantly to the understanding of almost all neurotransmitter systems. Worldwide, there are many plants that contain psychoactive properties, and people have been using them for ages. Psychoactive plant compounds may significantly alter how people perceive the world.
Compound Summary
CAS ID |
N/A |
||
INCHI KEY |
FWMPKHMKIJDEMJ-UHFFFAOYSA-N |
||
MOLECULAR WEIGHT |
194.23 |
||
MOLECULAR FORMULA |
C11H14O3 |
||
MOLECULAR MASS |
194.230 |
||
BIOLOGICAL SOURCE |
Sassafras albidum |
||
DATA SOURCE |
Afendi, F. M., Okada, T., Yamazaki, M., Hirai-Morita, A., Nakamura, Y., Nakamura, K., Ikeda, S., Takahashi, H., Altaf-Ul-Amin, M., Darusman, L. K., Saito, K., & Kanaya, S. (2012). KNApSAcK family databases: integrated metabolite-plant species databases for multifaceted plant research. Plant & cell physiology, 53(2), e1. https://doi.org/10.1093/pcp/pcr165 | ||
CHEMICAL CLASS OF COMPOUND |
Phenols |
||
NLRP3 DOCKING SCORE(Kcal/mol) |
-4.226 |
||
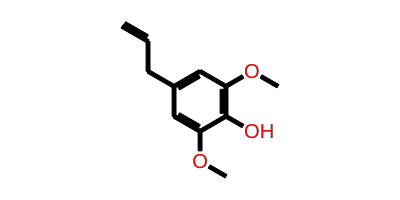
CANONICAL SMILES C=CCc1cc(OC)c(O)c(OC)c1
|
|||
BIOACTIVITY REPORTED FOR NEURODEGENERATIVE DISEASES
N/A
|
|||
SYNONYMS |
4-Allyl-2,6-dimethoxyphenol, 6627-88-9, Methoxyeugenol, 2,6-dimethoxy-4-prop-2-enylphenol, 4-Allylsyringol, 2,6-Dimethoxychavicol, Phenol, 2,6-dimethoxy-4-(2-propenyl)-, Phenol, 4-allyl-2,6-dimethoxy-, 2,6-Dimethoxy-4-allylphenol, 4-Allyl-2,6-dimetoxyphenol, 4-Hydroxy-3,5-dimethoxyallylbenzene, FEMA No. 3655, CHEBI:86562, 6-methoxyeugenol, UNII-8VF00YWP89, 2,6-dimethoxy-4-(2-propenyl)phenol, 8VF00YWP89, EINECS 229-600-2, NSC 16953, NSC 60246, NSC-16953, NSC-60246, Phenol, 4-(2-propenyl)-2,6-dimethoxy, 2,6-dimethoxy-4-(prop-2-en-1-yl)phenol, AI3-23057, Phenol,2,6-dimethoxy-4-(2-propen-1-yl)-, 4-(2-PROPENYL)SYRINGOL, DTXSID30216470, CHAVICOL, 2,6-DIMETHOXY-, Phenol,6-dimethoxy-, 4-ALLYL-2,6-DIMETHOXYPHENOL [FHFI], PHENOL, 2,6-DIMETHOXY-4-(2-PROPEN-1-YL)-, 4-(2-Propenyl)-2,6-dimethoxyphenol (4-allylsyringol), Phenol,6-dimethoxy-4-(2-propenyl)-, MFCD00008655, bmse010054, SCHEMBL293977, 4-allyl-2,6-dimethoxy-phenol, CHEMBL2059292, DTXCID30138961, NSC16953, NSC60246, 4-Allyl-2,6-dimethoxyphenol, 8CI, BDBM50242963, 4-(2-Propenyl)-2,6-dimethoxyphenol, AKOS015888171, 2,6-dimethoxy-4-(2-propenyl)-phenol, 2,6-Dimethoxy-4-(2-propenyl) phenol, 4-Allyl-2,6-dimethoxyphenol, >=95%, Phenol, 4-allyl-2,6-dimethoxy-(8CI), AS-36789, BP-10517, Phenol, 4-allyl-2,6-dimethoxy- (8CI), DB-054902, 2,6-Dimethoxy-4-(2-propenyl)phenol, 9CI, 4-Allyl-2,6-dimethoxyphenol, >=95%, FG, CS-0158007, NS00022639, Phenol, 2,6-dimethoxy-4-(2-propenyl)-(9CI), Phenol, 2,6-dimethoxy-4-(2-propenyl)- (9CI), Q27159248 |
||